Scientific Data
The VP.S ENCORE® is designed to improve transplant outcomes by addressing key limitations in current heart preservation and transport.
Its development focuses on expanding the donor pool, improving organ matching opportunities, and reducing the risk of graft rejection.
Human Data
Non-utilized human hearts from brain-dead donors ( n = 11) were obtained from the Texas Organ Sharing Alliance (TOSA), South Central Texas’ Organ Procurement Organization (OPO) and were preserved in the VP.S ENCORE® for 4, 6, and 8 hours or were kept in static cold storage (SCS). After preservation, hearts were placed in an isolated heart Langendorff model for reperfusion and evaluated for cardiac function.
Our results show that preserving non-utilized human hearts using the VP.S ENCORE® cardiac perfusion device enhances cardiac viability and demonstrates similar cardiac function to a healthy heart. Our findings imply that the VP.S ENCORE® device may provide a new paradigm in organ preservation for both standard and extended criteria hearts.
VP.S ENCORE® Preserved Human Hearts Not Suitable for Transplant
VP.S ENCORE® Heart on Langendorff Apparatus
Porcine Transplant Data
We have continued testing the VP.S ENCORE® at The Texas Heart Institute using a porcine heart transplant model. We recently completed an orthotopic heart transplant study where we preserved porcine hearts using both the standard of care (SoC), static cold storage and VP.S ENCORE®. After weaning off the bypass machine we monitored animals for an additional 6 hours.
Collected data demonstrates that in comparison to the SoC heart, the heart preserved in VP.S ENCORE® had lower duration on bypass, needed significantly less inotropic support, had better renal and cardiac function, and had significantly lower lactate expression.
Recent Animal Study Demonstrates VP.S Advantages vs. SoC
Porcine Orthotopic Heart Transplant Case (July-August 2023)
VP.S ENCORE® Heart Healthier Post Transplant
Compared to Standard of Care during 6-hour post Transplant follow-up
Porcine Preclinical Data
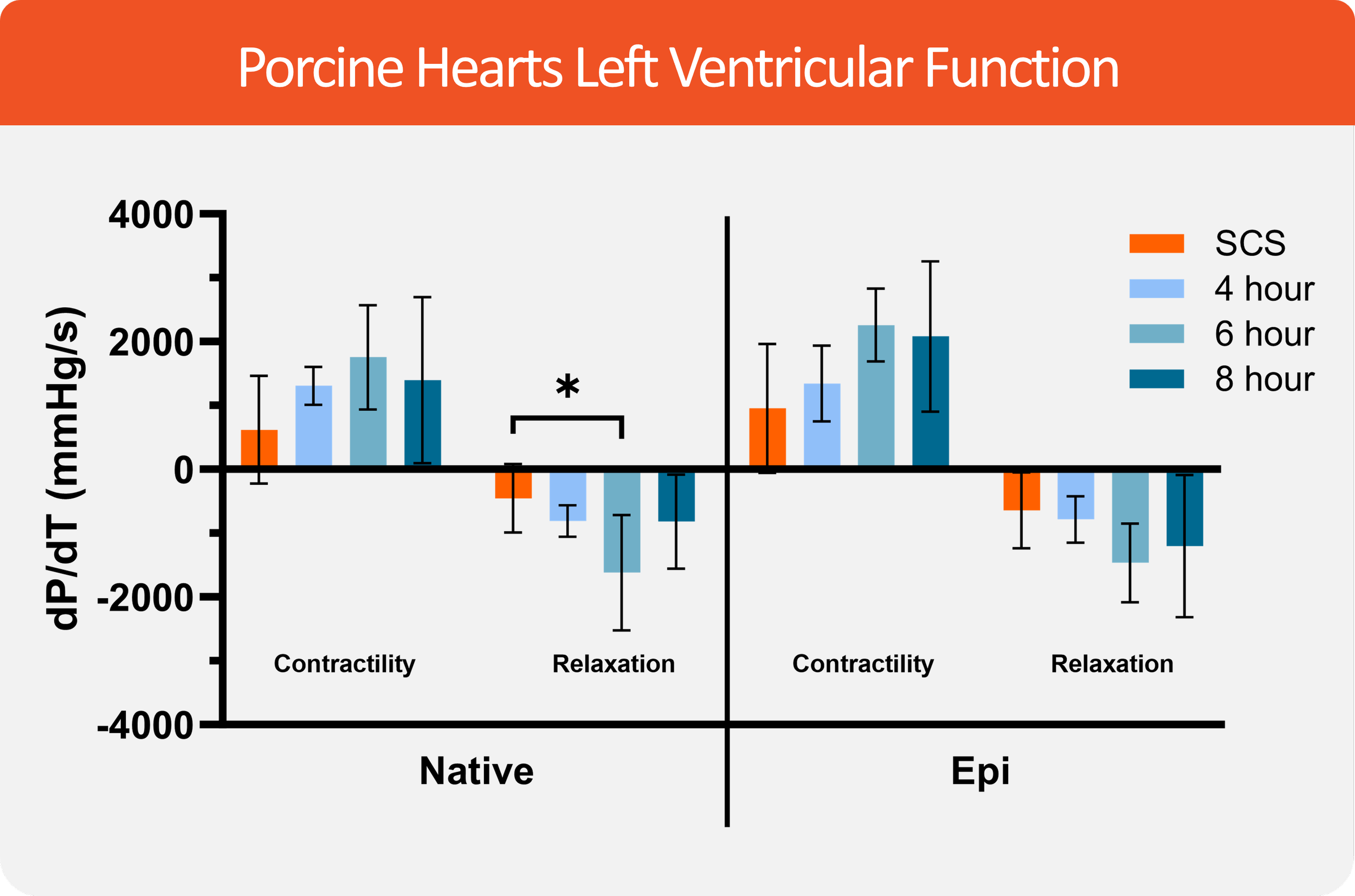
We tested the efficacy of the VP.S ENCORE® device in over 100 porcine hearts preserved for 4, 6, 8, and 24 hours. Our data demonstrates minimal or no edema after preservation, shift from anaerobic metabolism to aerobic metabolism, a significant reduction in inflammatory markers as assessed by gene expression assays, and significantly higher contractility compared to static cold storage hearts.
Our preclinical data demonstrates that the simple to use VP.S ENCORE® cardiac preservation device can serve as an alternative to the static cold storage.
VP.S ENCORE® Preserved Hearts Demonstrate Stable Weight
Porcine Orthotopic Heart Transplant Case (July-August 2023)
VP.S ENCORE® Demonstrates Improved LV Contractility vs. SCS
24-hour Porcine Heart vs 4-hour Control Static Cold Storage
Left: 4-Hour SCS
Right: 24-Hour VP.S ENCORE®
Hearts preserved in VP.S ENCORE® had significantly lower expression of inflammatory and cell death markers
Biopsy samples were taken post-preservation and gene expression was assessed by qRT-PCR
Lactate trends during different time points of perfusion
Hearts preserved with VP.S ENCORE® go from anaerobic metabolism (producing lactate) to aerobic metabolism (consuming lactate) and not exceeding 2mmom/L concentration throughout all experiments.
Note: Hearts measuring lactates higher than 5mmol/L can be rejected
Testimonials from Surgeons and Scientists Regarding VP.S ENCORE®
-
“Static cold storage with ice has been the gold standard for donor organ storage and transport since the early days of heart transplantation. Although practiced for decades, this mode of organ preservation is time limited to less than 4 hours and exposes the graft to cold end ischemic injury. ENCORE device mitigates both cold and ischemic injury and safely extends the period that the organ can be preserved outside of the body. In comparison to its competitors, it provides unrivaled portability and ease of use. Absence of the need for the whole blood as an oxygen carrier, ENCORE device stands out from other commercially available non ischemic heart preservation systems. I am certain this device will dramatically change the landscape of heart transplantation. I am particularly excited for the potential of the ENCORE device to improve the outcomes of transplanting the grafts from donation after circulatory death.”
-
“This approach may allow for better placement as well as function of these transplanted hearts. The hope is that through extended viability of the donor heart, a more precise immunologic matching may be achieved which will result in improved long-term survival of transplants, as well as better donor utilization. The donor utilization is now only about 35% of the hearts offered for transplant.”
-
“Put another way, that 35% organ utilization rate indicates that up to 65% of the hearts are not viable due to problems during transport, including lack of oxygen, nutrients, or circulation. The VP.S ENCORE® system changes that by supplying oxygen and nutrients at low temperatures and reducing inflammation and rejection in the recipients. This medical device will help to increase organ viability and therefore the organ pool available to hospitals around the United States. This will ultimately improve the long-term survival of organs for patients in need of a transplant.”